Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Therapeutic Effect and Mechanism of Keluoxin on Diabetic Retinopathy by Untargeted Metabolomics Analysis Based On UPLC-MS/MS
*Corresponding author: Xijun Wang, National Chinmedomics Research Center & National TCM Key Laboratory of Serum Pharmacochemistry, Department of Pharmaceutical Analysis, Heilongjiang University of Chinese Medicine, Harbin, China.
Received: April 04, 2022; Published: April 22, 2022
DOI: 10.34297/AJBSR.2022.16.002196
Abstract
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes and one of the leading causes of blindness worldwide. Keluoxin (KLX) is a Chinese patent medicine, can effectively improve DR, but its mechanism is not yet clear. This study aims to reveal the mechanism of KLX effect on DR through untargeted metabolomics. The diabetic retinopathy model was established in db/db mice, and the DR development process of the mice and the intervention effect of KLX on DR were evaluated by body weight, blood glucose, electroretinogram and histopathology. UPLC-MS/MS was used for urine metabolomic analysis, and Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) were used to identify DR urine biomarkers. Pathway analysis and literature reveal the mechanism by which KLX interferes with DR. KLX can significantly reduce body weight and blood glucose in db/db mice, improve retinal function and structure in db/db mice, and has an effective DR improvement effect. The results of urine metabolomics analysis showed that KLX could regulate the metabolic profile of db/db mice, making it tend to be close to the control group. A total of 69 DR urine biomarkers were identified, of which 50 were recalled by KLX. It was found that the KLX improved DR may be related to the regulation of tryptophan metabolism and arachidonic acid metabolism. We discuss the potential mechanism of KLX affects DR, which provides a scientific basis for the application of KLX and the treatment of DR.
Keywords: Keluoxin, Diabetic retinopathy, Urine, Untargeted metabolomics
Abbreviations: DR: Diabetic Retinopathy; KLX: Keluoxin; PCA: Principal Component Analysis; OPLS-DA: Orthogonal Partial Least Squares Discriminant Analysis; WHO: World Health Organization; HPLC: High-Performance Liquid Chromatography; UPLC-MS/ MS: Ultra-Performance Liquid Chromatography - Mass - Mass Spectrometry; CE: Collision Energy; CES: Collision Energy Spread; APCI: Atmospheric Pressure Chemical Ionization Source; POS: Positive; NEG: Negative; Metpa: Metabolomics Pathway Analysis; COX: Cyclooxygenase; LOX: Lipoxygenase; CYP: Cytochrome; PGI2: Prostaglandin I2; PGD2: Prostaglandin D2; PGB2: Prostaglandin B2; Eets: Epoxyeicosatrienoic Acids.
Introduction
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes and one of the leading causes of blindness worldwide [1]. The World Health Organization (WHO) estimates that diabetic retinopathy accounts for 15-17% of total blindness in Europe and the United States [2]. In diabetic patients, the prevalence of diabetic retinopathy is about one-third [3]. By 2030, there will be 191 million people with diabetic retinopathy [4]. Although the aetiology and pathology of DR have been extensively studied for half a century, there is still a lack of effective treatments [5,6]. Health care costs for people with diabetic retinopathy double compared to those without diabetes [7]. Therefore, any strategy that can treat and inhibit diabetic retinopathy is of great value to diabetic patients and society as a whole. Keluoxin (KLX) is a Chinese patent medicine, widely used in the treatment of diabetes and its complications in China [8-10]. Studies have shown that KLX can reduce blood glucose [8,11,12], inhibition of retinal microangiogenesis in db/db mice, reduced the thickness of the retinal ganglion layer and the inner plexiform layer, and improve retinal function [10,12], but its mechanism of action to improve diabetic retinopathy remains unclear.
Metabolomics provides the overall metabolic profile of a biological sample [13,14]. It is the analysis of a large number of endogenous small molecules [15-17]. There are two main analysis methods in metabolomics, targeted metabolomics and untargeted metabolomics [18-20]. Untargeted metabolomics aims to capture more metabolite information and is widely used in the identification of disease biomarkers [21-24], helping to improve our understanding of disease pathophysiology and mechanisms [25-28]. DR is a chronic metabolic disorder with complex pathogenesis [29,30], untargeted metabolomics can systematically explain the metabolic changes of DR and is a powerful tool to reveal the mechanism of KLX intervention in DR [31,32]. In this study, we investigated the effect of KLX on DR. UPLC-MS/MS-based untargeted metabolomic research method to identify urinary biomarkers in DR model mice. And from the perspective of systems biology, metabolomics was used to analyze the changes of KLX on DR biomarkers, revealing the mechanism of KLX's intervention in DR.
Methods
Instrument
Acquity™ UPLC liquid chromatography (Waters, USA); AB SCIEX Triple TOF™ 5600 + (SCIEX, USA); Ultrasonic cleaner (KQ-250 DB, Kunshan Ultrasonic Instrument Co., Ltd., China); Table centrifuge (sorvall ST 16R, Thermo Scientific, USA); Thermo Scientific 995 ultra-low temperature refrigerator (Thermo Scientific, USA), RetiMINER-C ophthalmic electrophysiology instrument (IRC, China); Transmission electron microscope (HT7700, Hitachi High-Technologies Corporation, Japan).
Chemicals and Materials
Acetonitrile (HPLC grade) was obtained from Fisher Scientific Corporation (Fisher, United States); ultrapure water was purchased from Watson’s Food & Beverage Co., Ltd (Guangzhou, China). KLX was provided by KANGHONG Pharmaceutical Company Ltd (Chengdu, China).
Animals and Sample Collecting
20 male db/db mice (mass 38 ± 2g) and 10 male db/m mice (mass 20 ± 2g) were provided by Nanjing Biomedical Research Institute of Nanjing University (NBRI). Mice had free access to food and drinking water under the following standard laboratory conditions(temperature 23 ± 3 °C, humidity 50 ± 10% and 12 h light/dark cycles). All mice were randomized into three groups: KLX group (db/db mice, n=10), model group (db/db mice, n=10) and control group (db/m mice, n=10).
Starting at 8 weeks, the KLX group was orally administered 780 mg/kg of KLX solution daily for 24 weeks. Control group and model group were given water (10mL/kg) for 24 weeks. Monitor body weight and fasting blood glucose every four weeks. This research was approved by the Ethical Committee of Heilongjiang University of Chinese Medicine and was conducted according to the principles expressed in the Declaration of Helsinki. Collection of 12 h night urine samples at 24 weeks of Keluoxin intervention, and then centrifuged for 10 min at 13000 rpm and 4°C, stored in the-80 °C frigerator for spare.
Electroretinogram
Before performing ERG, keeping the mice in a completely dark environment for 12 hours for dark adaptation. The mice were anesthetized with 0.3% sodium pentobarbital (30 mg kg-1), the cornea was surface anesthetized with lidocaine, and the pupils were dilated with tropicamide. Use RetiMINER-C ocular electrophysiological inspection platform to record electroretinogram according to ISCEV standard. The values for a and b-wave amplitudes and implicit times are obtained using an inbuilt analysis tool from RetiMINER-C.
Histopathology
The eyeballs of mice were fixed in glutaraldehyde solution at 4°C for 2 h. and then the retinas were separated, and ultrathin sections were prepared. The ultrastructure of retinal capillaries and visual cells were observed by HT7700 transmission electron microscope.
Pre-treatment of Urine Sample for Metabolomics
Urine samples (100 µL) were diluted 1:3 (vol/vol) using water, vortex for 60 seconds, centrifuge for 10 minutes (13000 rpm, 4°C), separate the supernatant, and screened with 0.22 µm filter membrane for analysis.
Chromatography Conditions
Chromatographic separation was performed by UPLC system (Acquity™ UPLC liquid chromatography, Waters, USA). Chromatographic separation was undertaken on an ACQUITY UHPLC BEH C18 (2.1 × 100 mm, 1.7 μm). The column temperature was maintained at 40 °C. The flow rate was 0.45 ml/min. The injected sample volume was 5 μL for each run. All the samples were kept at 4°C during the analysis. The optimal mobile phase containing a linear gradient elution program of 0.1% formic acid in acetonitrile (solvent A) and 0.1% formic acid in water (solvent B) was performed as follows: 0 min at 1%A; 0-6 min at 1-20% A; 6-6.5 min at 20-22% A; 6.5-10 min at 25-40% A; 10-10.5 min at 40-55% A; 10.5-11.5 min at 55-100% A.
Mass Spectrometry
The AB SCIEX Triple TOFTM 5600+ mass spectrometry system (High-definition mass spectrometry) was used to collect sample information and use DuoSprayTM electrospray ion source (ESI) source to operate under positive ion and negative ion mode respectively. The ion spray voltage floating (ISVF) 5500V(ESI+) and 4000V (ESI-), ion source temperature 600 °C, ion source gas 1 & 2 pressure 60 psi; and curtain gas pressure 25 psi. In TOFMS-IDA-MS/MS analysis, the mass range of positive and negative ion scanning is 50-1600 amu, and the accumulation times is 100 ms. Under the working mode of IDA (Information Dependent Analysis) triggered by Dynamic Background Subtract (DBS), eight ions with response intensity exceeding 100 CPS were scanned in each analyte. The accumulation times was 50 ms, the collision energy (CE) was 35eV and the collision energy spread (CES) was 15 eV. CDS (Calibrant Delivery System) uses atmospheric pressure chemical ionization source (APCI) and external standard correction method to adjust and correct MS and MS/MS automatically. The workstation is Analyst TF 1.6.2. The accurate mass acquired were processed by means of the elemental composition calculator incorporated in the Peak View® software (AB SCIEX).
Data Processing
All raw data imported into the Progenesis QI software for data dimension reduction and matrix acquisition. This software can automatically complete peak recognition, noise reduction, peak alignment, peak picking and other preprocessors, and finally the output matrix included the retention time, m/z value, and normalized peak area. Import this three-dimensional matrix information into EZinfo software for multivariate statistical analysis, use principal component analysis (PCA) to visually express the features of multivariate data in low-dimensional space to observe the distribution between groups. The VIP curve constructed with OPLS-DA selects ions that have an important contribution to distinguishing the model group from the control group (VIP>1) and have p-value (Student’s t-test) were less than 0.05 as potential biomarkers. Then, the structures of potential biomarkers were characterized by comparing their chromatographic and spectrometric data with Human Metabolome Database (HMDB, https://hmdb.ca/) and literature data. Finally, we calculated and evaluated significant pathways assisted by Kyoto Encyclopedia of Genes and Genomes database (KEGG, http://www.genome.jp/kegg/).
Result
Improvement Effect of KLX on db/db Mice
KLX can reduce fasting blood glucose and body weight in db/db mice and improve the implicit time and amplitudes of the electroretinogram a-wave and b-wave in model mice. Besides, KLX can improve retinal structure in db/db mice and has an effective DR improvement effect. This part of the results was discussed in our previous study [12].
Effects of KLX on Urine Metabolic Profile of DR Mice
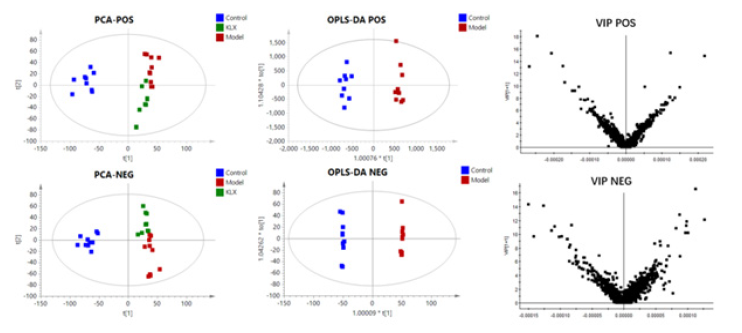
A quality control sample was tested in every 10 samples to ensure system stability. Metabolic profiles of 32-week urine samples were scanned in positive and negative ion mode (Figure 1). The UPLC-MS/MS data information was aligned and normalized using QI software. Then imported into EZinfo software for multivariate statistical analysis, PCA plots revealed a separate clustering between control, model group and KLX group. Compared with the model group, the KLX group had a tendency to be close to the control group, no obvious outlier samples were found (Figure 2).
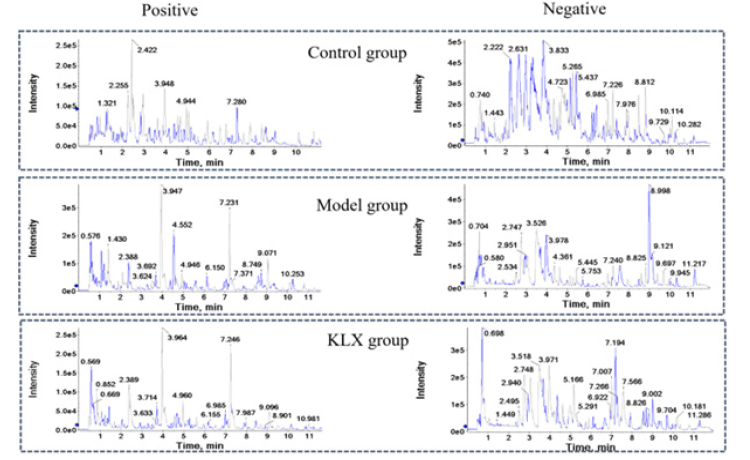
Figure 1: The BPI Chromatograms of Urine samples of Control Group, Model Group and KLX Group in positive and Negative ion Mode of UHPLC-Q-TOF/MS(IDA).
Urine Biomarkers in DR Mice
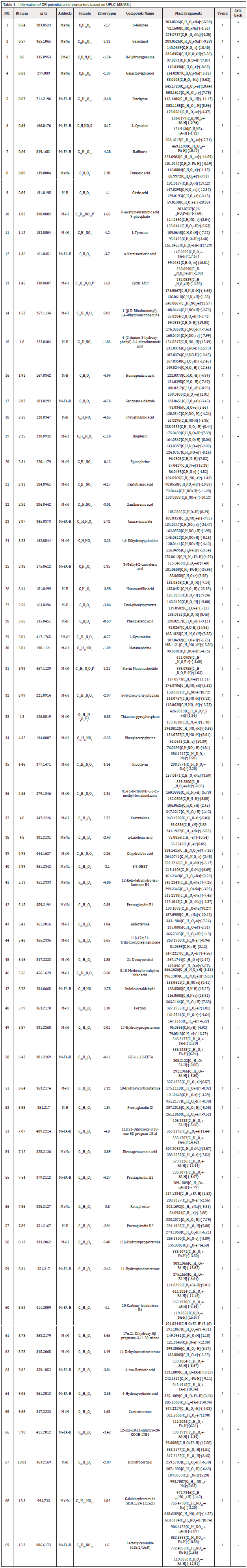
The OPLS-DA plots shown a clear separation of between control group and model the group was obtained under both positive ion mode and negative ion mode (Figure 2). According to OPLS-DA model and p-value of Student's t-test, VIP> 1 and p < 0.05 as screening criteria for differential metabolites. The structures of potential biomarkers were characterized by comparing their chromatographic and spectrometric data with Human Metabolome Database (HMDB, https://hmdb.ca/) and literature data. A total of 69 potential urinary biomarkers for diabetic retinopathy were identified, mainly including 14 steroids (cortexolone, aldosterone, and cortisol etc.), 9 eicosanoids (8,9-DHET, 15H-11,12-EETA, prostaglandin I2, prostaglandin D2, and prostaglandin B2 etc.), 3 Phenylacetic acids (homovanillic acid, homogentisic acid, and phenylacetic acid), 3 fatty acids and conjugates (alpha-ketoisovaleric acid, alpha-Linolenic acid, and eicosapentaenoic acid). Information on potential biomarkers is listed in Table 1. The levels of potential biomarkers in the model group and control group are shown in Figure 3.
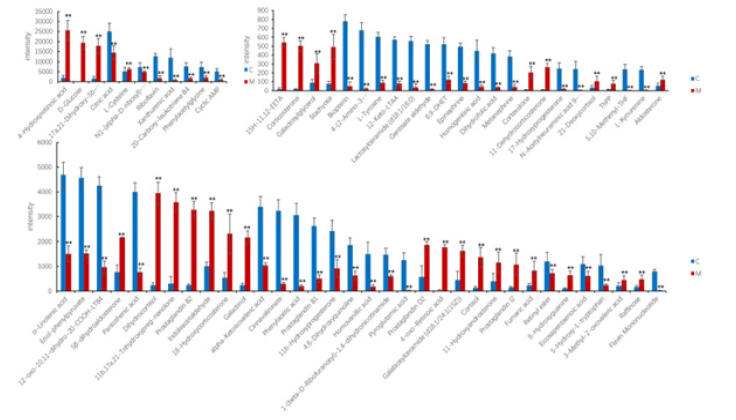
Figure 3: Comparison of biomarker levels between control group and model group. The red histogram indicates model group, the blue histogram indicates control group. *P<0.05 VS. control; **P<0.01 VS. control; #P<0.05 VS. model, ##P<0.01 VS. model.
MetPA (Metabolomics Pathway Analysis) was performed on 69 characterized urinary biomarkers of diabetic retinopathy. The results showed that diabetic retinopathy involved changes in the riboflavin metabolism, thiamine metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, alpha-Linolenic acid metabolism, steroid hormone biosynthesis, tyrosine metabolism, tryptophan metabolism (Figure 4).
Effects of KLX on Potential Urine Biomarkers
By analyzing the levels of potential biomarkers in each group, KLX was able to recall 50 of the 69 urine biomarkers, and the level comparison of the recalled biomarkers is shown in the Figure 5. Six biomarkers of tryptophan metabolism were recalled, including L-kynurenine, 5-hydroxy-L-tryptophan, indoleacetaldehyde, 4,6-dihydroxyquinoline, cinnavalininate, and 4-(2-amino-3-hydroxyphenyl)- 2,4-dioxobutanoic acid; 6 biomarkers of arachidonic acid metabolism were recalled, including 12-keto-LTA4, prostaglandin I2, prostaglandin D2, prostaglandin B2, 8,9-DHET, and 15H-11,12-EETA; 6 biomarkers of galactose metabolism were recalled, including D-glucose, raffinose, stachyose, α-D-glucuse-6P, galactosyl-glycerol, and galactinol. The information of KLX callback markers is shown in Table 1.
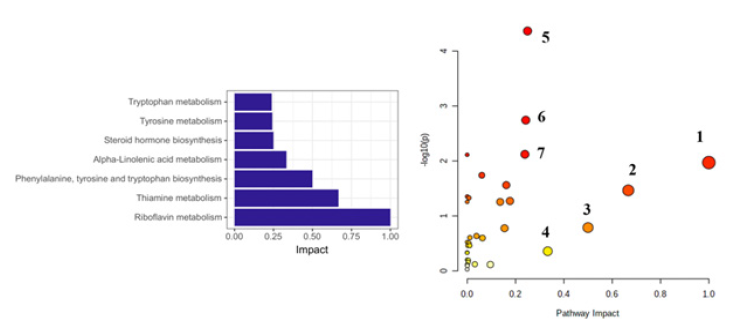
Figure 4: Pathway analysis of potential urinary biomarkers for DR. 1, riboflavin metabolism; 2, thiamine metabolism; 3, phenylalanine, tyrosine and tryptophan biosynthesis; 4, alpha-Linolenic acid metabolism; 5, steroid hormone biosynthesis; 6, tyrosine metabolism; 7, tryptophan metabolism.
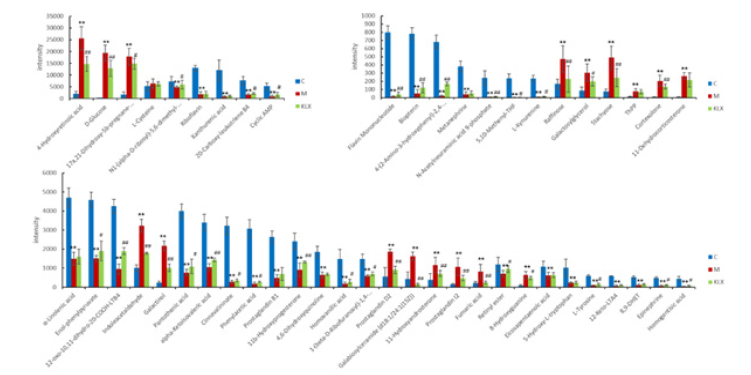
Figure 5: Comparison of biomarker levels recalled by KLX. The red histogram indicates model group, the green histogram indicates control group, and the blue histogram indicates KLX group. *P<0.05 VS. control; **P<0.01 VS. control; #P<0.05 VS. model, ##P<0.01 VS. model.
Mechanism analysis of KLX Improving DR
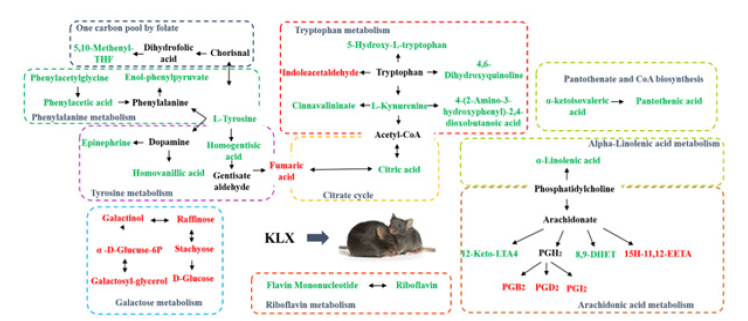
Analysis by HMDB, KEGG and the metaboanalyst platform showed that KLX may improve DR by regulating metabolic pathways such as tryptophan metabolism, arachidonic acid metabolism, riboflavin metabolism, pantothenate and CoA biosynthesis, galactose metabolism, tyrosine metabolism, phenylalanine metabolism, one carbon pool by folate, and alpha-Linolenic acid metabolism, and the mechanism of KLX intervening in DR is shown in the Figure 6.
Discussion
Diabetic retinopathy is one of the microvascular complications of diabetes and has been recognized as the leading cause of blindness worldwide [33]. The main pathogenesis of DR is the accumulation of glycation end products, oxidative stress, polyol pathway and protein kinase C (PKC) activation [34,35]. There is no effective treatment for diabetic retinopathy, and early diagnosis and drug intervention can effectively slow the progression of the disease. Therefore, it is important to identify biomarkers associated with diabetic retinopathy. In this study, a total of 69 urinary biomarkers for DR were discovered and identified, and enrichment analysis indicated that DR was associated with altered metabolic pathways such as steroid hormone biosynthesis, tryptophan metabolism, phenylalanine metabolism, tyrosine metabolism, and riboflavin metabolism. Through metabolomics research methods, we found that KLX improves with diabetic retinopathy by regulating multiple metabolic pathways, including tryptophan metabolism, arachidonic acid metabolism.
Tryptophan is an essential amino acid for maintaining cell proliferation and differentiation, and kynurenine and 5-hydroxytryptamine are the two main metabolic pathways of tryptophan [36,37]. The urine biomarkers for diabetic retinopathy identified in this study, kynurenine and its metabolites cinnavalininate and 4-(2-Amino-3-hydroxyphenyl)-2,4-dioxobutanoic acid were significantly reduced, which revealed a biological abnormality of the kynurenine pathway, and the imbalance of the kynurenine pathway could lead to endothelial cell apoptosis and subsequent endothelial dysfunction [38,39]. Kynurenine is an endogenous agonist of aryl hydrocarbon receptors, and after binding to aryl hydrocarbon receptors, it causes the expression of a series of target genes, blocks T cell proliferation and induces T cell death [40]. Furthermore, kynurenine suppress the activity of antigen-presenting cells such as dendritic cells, monocytes, and macrophages in mice [41,42], which in turn suppresses the immune response and ameliorates nerve damage. Keluoxin can adjust the level of kynurenine which is the potential mechanism of Keluoxin affecting diabetic retinopathy. The effect of arachidonic acid metabolism on diabetes and its complications is critical [43]. High glucose environment can promote the release of arachidonic acid from platelet membrane phospholipids [44]. Arachidonic acid can be further metabolized by cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP) pathways [45,46]. Prostaglandins are generated from arachidonic acid via the cyclooxygenase pathway, which can cause inflammatory signals to affect the development of DR [46-48]. In our study, the levels of prostaglandin I2, prostaglandin D2, and prostaglandin B2 were elevated in the model group, and the KLX recalled the levels of these biomarkers. 8,9DHET is an epoxyeicosatrienoic acids (EETs) generated by arachidonic acid catalyzed by cytochrome P450 Xu et al. [49]. studied the relationship between EETs and insulin resistance and found that EETs can stimulate islet cells to secrete insulin, improve glucose homeostasis, and inhibit islet cell apoptosis, thereby inhibiting the occurrence of diabetes and its complications [50]. In this experiment, KLX was able to recall 8,9DHET, which was reduced in the model group.
Conclusion
In this study, DR model was established in db/db mice, and we identified 69 DR urinary biomarkers, KLX can recall 50 of them and regulate metabolic pathways such tryptophan metabolism and arachidonic acid metabolism. These reveal the molecular mechanism of KLX regulated DR., KLX has great prospects as an effective drug to improve DR.
Acknowledgements
This work was supported by the Natural Science Foundation of Heilongjiang Province (LH2020H095). All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rasmussen M (2013) Aerococci and aerococcal infections. The Journal of infection 66(6): 467-474.
- Rasmussen M (2016) Aerococcus: an increasingly acknowledged human pathogen. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 22(1): 22-27.
- Lewis AL, Gilbert NM (2020) Roles of the vagina and the vaginal microbiota in urinary tract infection: evidence from clinical correlations and experimental models. GMS Infect Dis 8:
- Chang Min H, Kyeong Min J, Ji Hoon J, Yoo Jin L, Bong Soo P, et al. (2016) Urosepsis with Aerococcus urinae in a Patient with Complicated Urinary Tract Infection. Korean J Med 91(2): 229-232.
- Higgins A, Garg T (2017) Aerococcus urinae: An Emerging Cause of Urinary Tract Infection in Older Adults with Multimorbidity and Urologic Cancer. Urology case reports 13: 24-25.
- Meletis G, Chatzidimitriou D, Tsingerlioti F, Chatzopoulou F, Tzimagiorgis G (2017) An initially unidentified case of urinary tract infection due to Aerococcus urinae. The new microbiologica 40(3): 221-222.
- Skalidis T, Papaparaskevas J, Konstantinou D, Kapolou E, Falagas ME, et al. (2017) Aerococcus urinae, a cause of cystitis with malodorous urine in a child: clinical and microbiological challenges. JMM case reports 4(2): e005083.
- Schempf TBS, Beg HMD, Tenner CMD (2018) Aerococcus urinae: An under-recognized cause of UTI in internal medicine. Curr Res Integr Med 3(S1): 11-12.
- Nauka P, Durst M (2019) Septicemia from urinary tract infection with negative urinary culture: Aerococcus urinae in an older adult. Chest 156(4): A2137.
- Cai E, Pearson S, Linnebur S, Fixen D (2020) Treatment of Aerococcus Urinae in an Older Adult in Living Liver Donors. Ann Clin Case Rep 2(1): 1007.
- Otero Colón J, Farraj KL, Desai Z (2022) An Uncommon Cause of Urinary Tract Infections: A Case Report. Cureus 14(3): e23325.
- Higgins A, Garg T (2017) Aerococcus urinae: An Emerging Cause of Urinary Tract Infection in Older Adults with Multimorbidity and Urologic Cancer. Urology case reports13: 24-25.
- Sahu KK, Lal A, Mishra AK, Abraham GM (2020) Aerococcus-Related Infections and their Significance: A 9-Year Retrospective Study. Journal of microscopy and ultrastructure 9(1): 18-25.
- Yaban B, Kikhney J, Musci M, Petrich A, Schmidt J, (2020) Aerococcus urinae - A potent biofilm builder in endocarditis. PLoS One 15(4): e0231827.
- Hilt EE, Putonti, Thomas White K, Lewis AL, Visick KL, et al. (2020) Aerococcus urinae Isolated from Women with Lower Urinary Tract Symptoms: In Vitro Aggregation and Genome Analysis. Journal of bacteriology 202(13): e00170-20.
- Gilbert NM, Choi B, Du J, Collins C, Lewis A L, et al. (2021). A mouse model displays host and bacterial strain differences in Aerococcus urinae urinary tract infection. Biology open 10(8): bio058931.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.